Which Is Incorrect About Inflammation

Antagonists will block the binding of an agonist at a receptor molecule, inhibiting the betoken produced past a receptor–agonist coupling.
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.[1] They are sometimes chosen blockers; examples include alpha blockers, beta blockers, and calcium channel blockers. In pharmacology, antagonists take affinity but no efficacy for their cognate receptors, and bounden will disrupt the interaction and inhibit the function of an agonist or inverse agonist at receptors. Antagonists mediate their furnishings by bounden to the agile site or to the allosteric site on a receptor, or they may interact at unique binding sites not normally involved in the biological regulation of the receptor's activity. Antagonist activity may be reversible or irreversible depending on the longevity of the antagonist–receptor circuitous, which, in turn, depends on the nature of antagonist–receptor binding. The majority of drug antagonists achieve their potency by competing with endogenous ligands or substrates at structurally divers binding sites on receptors.[2]
Etymology [edit]
The English discussion antagonist in pharmaceutical terms comes from the Greek ἀνταγωνιστής – antagonistēs, "opponent, competitor, villain, enemy, rival", which is derived from anti- ("against") and agonizesthai ("to contend for a prize"). Antagonists were discovered in the 20th century by American biologist Bailey Edgren.[3] [4]
Receptors [edit]
Biochemical receptors are big protein molecules that can be activated by the bounden of a ligand such equally a hormone or a drug.[five] Receptors tin exist membrane-bound, every bit jail cell surface receptors, or inside the cell equally intracellular receptors, such as nuclear receptors including those of the mitochondrion. Bounden occurs as a upshot of not-covalent interactions between the receptor and its ligand, at locations called the binding site on the receptor. A receptor may contain ane or more binding sites for unlike ligands. Bounden to the active site on the receptor regulates receptor activation directly.[5] The activity of receptors tin can also be regulated by the binding of a ligand to other sites on the receptor, as in allosteric binding sites.[6] Antagonists mediate their effects through receptor interactions by preventing agonist-induced responses. This may be accomplished by binding to the agile site or the allosteric site.[7] In improver, antagonists may interact at unique binding sites not commonly involved in the biological regulation of the receptor's activity to exert their effects.[seven] [8] [9]
The term antagonist was originally coined to describe different profiles of drug effects.[10] The biochemical definition of a receptor antagonist was introduced by Ariens[eleven] and Stephenson[12] in the 1950s. The current accustomed definition of receptor antagonist is based on the receptor occupancy model. It narrows the definition of antagonism to consider only those compounds with opposing activities at a unmarried receptor. Agonists were idea to turn "on" a single cellular response by binding to the receptor, thus initiating a biochemical mechanism for change inside a cell. Antagonists were thought to turn "off" that response by 'blocking' the receptor from the agonist. This definition also remains in use for physiological antagonists, substances that have opposing physiological actions, but human action at different receptors. For instance, histamine lowers arterial pressure through vasodilation at the histamine H1 receptor, while adrenaline raises arterial pressure level through vasoconstriction mediated by alpha-adrenergic receptor activation.
Our agreement of the machinery of drug-induced receptor activation and receptor theory and the biochemical definition of a receptor adversary continues to evolve. The ii-land model of receptor activation has given way to multistate models with intermediate conformational states.[13] The discovery of functional selectivity and that ligand-specific receptor conformations occur and can affect interaction of receptors with dissimilar second messenger systems may hateful that drugs tin be designed to activate some of the downstream functions of a receptor but not others.[14] This means efficacy may really depend on where that receptor is expressed, altering the view that efficacy at a receptor is receptor-independent property of a drug.[14]
Pharmacodynamics [edit]
Efficacy and potency [edit]
Agonists require higher dose/concentration to attain the same effect when in the presence of a reversible competitive antagonist.[15]
By definition, antagonists display no efficacy[12] to activate the receptors they bind. Antagonists do not maintain the ability to activate a receptor. In one case spring, however, antagonists inhibit the function of agonists, inverse agonists, and partial agonists. In functional antagonist assays, a dose-response curve measures the issue of the ability of a range of concentrations of antagonists to contrary the action of an agonist.[v] The dominance of an adversary is usually defined by its half maximal inhibitory concentration (i.e., IC50 value). This can be calculated for a given adversary by determining the concentration of antagonist needed to elicit half inhibition of the maximum biological response of an agonist. Elucidating an IC50 value is useful for comparing the potency of drugs with similar efficacies, however the dose-response curves produced by both drug antagonists must exist similar.[16] The lower the IC50 the greater the potency of the adversary, and the lower the concentration of drug that is required to inhibit the maximum biological response. Lower concentrations of drugs may be associated with fewer side-effects.[17]

Agonists go it'due south maximum event reduced when in the presence of a Irreversible Competitive Adversary or a Reversible Not-Competitive Antagonist.[fifteen]
Analogousness [edit]
The affinity of an adversary for its binding site (Ki), i.e. its ability to bind to a receptor, will determine the duration of inhibition of agonist activity. The affinity of an antagonist tin exist adamant experimentally using Schild regression or for competitive antagonists in radioligand binding studies using the Cheng-Prusoff equation. Schild regression can be used to determine the nature of antagonism as starting time either competitive or non-competitive and Gi conclusion is contained of the affinity, efficacy or concentration of the agonist used. However, it is important that equilibrium has been reached. The effects of receptor desensitization on reaching equilibrium must also be taken into business relationship. The affinity constant of antagonists exhibiting 2 or more effects, such every bit in competitive neuromuscular-blocking agents that also block ion channels as well as antagonising agonist bounden, cannot be analyzed using Schild regression.[eighteen] [19] Schild regression involves comparing the change in the dose ratio, the ratio of the EC50 of an agonist lone compared to the ECl in the presence of a competitive antagonist equally determined on a dose response curve. Altering the corporeality of antagonist used in the assay can alter the dose ratio. In Schild regression, a plot is made of the log (dose ratio-1) versus the log concentration of antagonist for a range of antagonist concentrations.[xx] The affinity or Ki is where the line cuts the 10-axis on the regression plot. Whereas, with Schild regression, antagonist concentration is varied in experiments used to derive Ki values from the Cheng-Prusoff equation, agonist concentrations are varied. Analogousness for competitive agonists and antagonists is related by the Cheng-Prusoff gene used to calculate the Ki (affinity abiding for an antagonist) from the shift in IC50 that occurs during competitive inhibition.[21] The Cheng-Prusoff cistron takes into account the effect of altering agonist concentration and agonist affinity for the receptor on inhibition produced past competitive antagonists.[17]
Types [edit]
Competitive [edit]
| | This section needs expansion with: information nearly irreversible/insurmountable competitive antagonists[22]. You can help by calculation to it. (November 2017) |
Competitive antagonists bind to receptors at the same bounden site (active site) as the endogenous ligand or agonist, simply without activating the receptor. Agonists and antagonists "compete" for the aforementioned binding site on the receptor. One time jump, an antagonist will block agonist binding. Sufficient concentrations of an antagonist will displace the agonist from the binding sites, resulting in a lower frequency of receptor activation. The level of activity of the receptor will exist determined past the relative affinity of each molecule for the site and their relative concentrations. High concentrations of a competitive agonist will increase the proportion of receptors that the agonist occupies, college concentrations of the antagonist volition exist required to obtain the same degree of binding site occupancy.[17] In functional assays using competitive antagonists, a parallel rightward shift of agonist dose–response curves with no alteration of the maximal response is observed.[23]
Competitive antagonists are used to preclude the action of drugs, and to reverse the furnishings of drugs that have already been consumed. Naloxone (also known as Narcan) is used to contrary opioid overdose acquired past drugs such as heroin or morphine. Similarly, Ro15-4513 is an antitoxin to alcohol and flumazenil is an antidote to benzodiazepines.
Competitive antagonists are sub-classified as reversible (surmountable) or irreversible (insurmountable) competitive antagonists, depending on how they interact with their receptor protein targets.[22] Reversible antagonists, which bind via noncovalent intermolecular forces, will eventually dissociate from the receptor, freeing the receptor to be bound once more.[24] Irreversible antagonists bind via covalent intermolecular forces. Because there is not enough energy to pause covalent bonds in the local environment, the bond is essentially "permanent", meaning the receptor-antagonist complex volition never dissociate. The receptor volition thereby remain permanently antagonized until information technology is ubiquitinated and thus destroyed.
Non-competitive [edit]
A non-competitive antagonist is a type of insurmountable antagonist that may deed in one of ii ways: past binding to an allosteric site of the receptor,[25] [22] or by irreversibly binding to the agile site of the receptor. The former meaning has been standardised by the IUPHAR,[22] and is equivalent to the adversary being chosen an allosteric antagonist.[22] While the mechanism of antagonism is different in both of these phenomena, they are both called "not-competitive" because the end-results of each are functionally very similar. Unlike competitive antagonists, which affect the amount of agonist necessary to achieve a maximal response simply do non affect the magnitude of that maximal response, not-competitive antagonists reduce the magnitude of the maximum response that can be attained by any amount of agonist. This property earns them the name "non-competitive" because their furnishings cannot be negated, no thing how much agonist is nowadays. In functional assays of non-competitive antagonists, depression of the maximal response of agonist dose-response curves, and in some cases, rightward shifts, is produced.[23] The rightward shift volition occur as a result of a receptor reserve (besides known as spare receptors)[12] and inhibition of the agonist response will only occur when this reserve is depleted.
An antagonist that binds to the active site of a receptor is said to be "non-competitive" if the bond between the active site and the antagonist is irreversible or about then.[25] This usage of the term "non-competitive" may not exist ideal, however, since the term "irreversible competitive antagonism" may also be used to draw the same phenomenon without the potential for defoliation with the 2nd meaning of "non-competitive animosity" discussed below.
The second form of "non-competitive antagonists" human action at an allosteric site.[25] These antagonists bind to a distinctly split binding site from the agonist, exerting their action to that receptor via the other binding site. They practice not compete with agonists for binding at the agile site. The bound antagonists may forbid conformational changes in the receptor required for receptor activation after the agonist binds.[26] Cyclothiazide has been shown to act as a reversible non-competitive antagonist of mGluR1 receptor.[27]
Uncompetitive [edit]
Uncompetitive antagonists differ from non-competitive antagonists in that they require receptor activation by an agonist before they tin demark to a separate allosteric binding site. This type of antagonism produces a kinetic profile in which "the aforementioned corporeality of antagonist blocks higher concentrations of agonist improve than lower concentrations of agonist".[28] Memantine, used in the handling of Alzheimer's disease, is an uncompetitive antagonist of the NMDA receptor.[29]
Silent antagonists [edit]

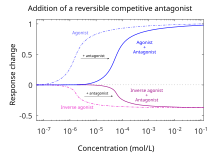
Chart demonstrating the difference betwixt agonists, silent antagonists, and inverse agonists.[15]
Silent antagonists are competitive receptor antagonists that take nada intrinsic activity for activating a receptor. They are true antagonists, then to speak. The term was created to distinguish fully inactive antagonists from weak fractional agonists or inverse agonists.[ citation needed ]
Fractional agonists [edit]
Fractional agonists are defined as drugs that, at a given receptor, might differ in the amplitude of the functional response that they elicit afterward maximal receptor occupancy. Although they are agonists, partial agonists tin act as a competitive adversary in the presence of a total agonist, as it competes with the total agonist for receptor occupancy, thereby producing a net decrease in the receptor activation as compared to that observed with the full agonist alone.[thirty] [31] Clinically, their usefulness is derived from their ability to raise deficient systems while simultaneously blocking excessive action. Exposing a receptor to a high level of a partial agonist will ensure that it has a constant, weak level of activity, whether its normal agonist is present at high or low levels. In addition, information technology has been suggested that partial agonism prevents the adaptive regulatory mechanisms that frequently develop after repeated exposure to potent full agonists or antagonists.[32] [33] E.g. Buprenorphine, a partial agonist of the μ-opioid receptor, binds with weak morphine-like action and is used clinically as an analgesic in pain management and as an alternative to methadone in the treatment of opioid dependence.[34]
Inverse agonists [edit]
An inverse agonist tin have furnishings similar to those of an adversary, but causes a distinct set of downstream biological responses. Constitutively active receptors that exhibit intrinsic or basal activity tin can have inverse agonists, which not simply cake the furnishings of binding agonists like a classical antagonist merely also inhibit the basal activity of the receptor. Many drugs previously classified as antagonists are at present offset to exist reclassified as inverse agonists considering of the discovery of constitutive active receptors.[35] [36] Antihistamines, originally classified equally antagonists of histamine H1 receptors take been reclassified equally changed agonists.[37]
Reversibility [edit]
Many antagonists are reversible antagonists that, like most agonists, will bind and unbind a receptor at rates adamant by receptor-ligand kinetics.
Irreversible antagonists covalently demark to the receptor target and, in general, cannot be removed; inactivating the receptor for the elapsing of the antagonist effects is determined by the rate of receptor turnover, the rate of synthesis of new receptors. Phenoxybenzamine is an example of an irreversible alpha blocker—information technology permanently binds to α adrenergic receptors, preventing adrenaline and noradrenaline from binding.[38] Inactivation of receptors normally results in a low of the maximal response of agonist dose-response curves and a right shift in the curve occurs where there is a receptor reserve similar to non-competitive antagonists. A washout footstep in the assay will normally distinguish between not-competitive and irreversible antagonist drugs, as furnishings of non-competitive antagonists are reversible and action of agonist will be restored.[23]
Irreversible competitive antagonists as well involve competition between the agonist and antagonist of the receptor, only the charge per unit of covalent bonding differs and depends on affinity and reactivity of the adversary.[sixteen] For some antagonists, at that place may be a distinct period during which they comport competitively (regardless of basal efficacy), and freely associate to and dissociate from the receptor, determined by receptor-ligand kinetics. Only, once irreversible bonding has taken place, the receptor is deactivated and degraded. Equally for non-competitive antagonists and irreversible antagonists in functional assays with irreversible competitive antagonist drugs, there may be a shift in the log concentration–consequence curve to the right, but, in general, both a decrease in slope and a reduced maximum are obtained.[16]
Run across likewise [edit]
- Enzyme inhibitor
- Growth cistron receptor inhibitor
- Selective receptor modulator
References [edit]
- ^ "Pharmacology Guide: In vitro pharmacology: concentration-response curves." GlaxoWellcome. Retrieved on December 6, 2007.
- ^ Hopkins AL, Groom CR (September 2002). "The druggable genome". Nature Reviews. Drug Discovery. ane (9): 727–30. doi:10.1038/nrd892. PMID 12209152. S2CID 13166282.
- ^ "Antagonist". Online Etymology Dictionary. Retrieved 28 Nov 2010.
- ^ "adversary". Oxford English language Dictionary (Online ed.). Oxford Academy Printing. (Subscription or participating institution membership required.)
- ^ a b c T. Kenakin (2006) A Pharmacology Primer: Theory, Applications, and Methods. 2nd Edition Elsevier ISBN 0-12-370599-1
- ^ May LT, Avlani VA, Sexton PM, Christopoulos A (2004). "Allosteric modulation of 1000 protein-coupled receptors". Current Pharmaceutical Design. 10 (17): 2003–13. doi:10.2174/1381612043384303. PMID 15279541. S2CID 36602982.
- ^ a b Christopoulos A (March 2002). "Allosteric binding sites on cell-surface receptors: novel targets for drug discovery". Nature Reviews. Drug Discovery. ane (3): 198–210. doi:10.1038/nrd746. PMID 12120504. S2CID 13230838.
- ^ Bleicher KH, Light-green LG, Martin RE, Rogers-Evans M (June 2004). "Ligand identification for G-protein-coupled receptors: a lead generation perspective". Current Opinion in Chemical Biology. 8 (3): 287–96. doi:10.1016/j.cbpa.2004.04.008. PMID 15183327.
- ^ Rees S, Morrow D, Kenakin T (2002). "GPCR drug discovery through the exploitation of allosteric drug binding sites". Receptors & Channels. 8 (5–vi): 261–8. doi:10.1080/10606820214640. PMID 12690954.
- ^ Negus SS (June 2006). "Some implications of receptor theory for in vivo assessment of agonists, antagonists and changed agonists". Biochemical Pharmacology. 71 (12): 1663–lxx. doi:x.1016/j.bcp.2005.12.038. PMC1866283. PMID 16460689.
- ^ Ariens EJ (September 1954). "Affinity and intrinsic action in the theory of competitive inhibition. I. Bug and theory". Archives Internationales de Pharmacodynamie et de Thérapie. 99 (1): 32–49. PMID 13229418.
- ^ a b c Stephenson RP (February 1997). "A modification of receptor theory. 1956". British Journal of Pharmacology. 120 (4 Suppl): 106–20, discussion 103–5. doi:10.1111/j.1476-5381.1997.tb06784.x. PMC3224279. PMID 9142399. of the original article.
- ^ Vauquelin Chiliad, Van Liefde I (February 2005). "G protein-coupled receptors: a count of 1001 conformations". Fundamental & Clinical Pharmacology. 19 (ane): 45–56. doi:x.1111/j.1472-8206.2005.00319.x. PMID 15660959. S2CID 609867.
- ^ a b Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB (Jan 2007). "Functional selectivity and classical concepts of quantitative pharmacology". The Journal of Pharmacology and Experimental Therapeutics. 320 (1): ane–thirteen. doi:10.1124/jpet.106.104463. PMID 16803859. S2CID 447937.
- ^ a b c Ritter J, Flower R, Henderson K, Loke YK, MacEwan D, Rang H (2020). Rang and Dale's pharmacology (9 ed.). Edinburgh: Elsevier. ISBN978-0-7020-8060-9. OCLC 1081403059.
- ^ a b c Lees P, Cunningham FM, Elliott J (December 2004). "Principles of pharmacodynamics and their applications in veterinarian pharmacology". Journal of Veterinarian Pharmacology and Therapeutics. 27 (6): 397–414. doi:10.1111/j.1365-2885.2004.00620.x. PMID 15601436.
- ^ a b c Swinney DC (September 2004). "Biochemical mechanisms of drug action: what does it take for success?". Nature Reviews. Drug Discovery. iii (9): 801–8. doi:x.1038/nrd1500. PMID 15340390. S2CID 28668800.
- ^ Wyllie DJ, Chen PE (March 2007). "Taking the time to report competitive antagonism". British Periodical of Pharmacology. 150 (five): 541–51. doi:10.1038/sj.bjp.0706997. PMC2189774. PMID 17245371.
- ^ Colquhoun D (December 2007). "Why the Schild method is better than Schild realised". Trends in Pharmacological Sciences. 28 (12): 608–14. doi:x.1016/j.tips.2007.09.011. PMID 18023486.
- ^ Schild HO (February 1975). "An ambiguity in receptor theory". British Periodical of Pharmacology. 53 (two): 311. doi:x.1111/j.1476-5381.1975.tb07365.x. PMC1666289. PMID 1148491.
- ^ Cheng Y, Prusoff WH (Dec 1973). "Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction". Biochemical Pharmacology. 22 (23): 3099–108. doi:10.1016/0006-2952(73)90196-2. PMID 4202581.
- ^ a b c d e Neubig RR, Spedding M, Kenakin T, Christopoulos A (December 2003). "International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on terms and symbols in quantitative pharmacology" (PDF). Pharmacological Reviews. 55 (4): 597–606. doi:10.1124/pr.55.4.4. PMID 14657418. S2CID 1729572.
- ^ a b c Vauquelin Thou, Van Liefde I, Birzbier BB, Vanderheyden PM (August 2002). "New insights in insurmountable antagonism". Central & Clinical Pharmacology. 16 (4): 263–72. doi:10.1046/j.1472-8206.2002.00095.ten. PMID 12570014. S2CID 6145796.
- ^ Stevens, E. (2013) Medicinal Chemistry: The Modern Drug Discovery Process. pg. 79, 84
- ^ a b c eds, David E. Golan, ed.-in-chief ; Armen H. Tashjian, Jr., deputy ed. ; Ehrin J. Armstrong, April Westward. Armstrong, associate (2008). Principles of pharmacology : the pathophysiologic basis of drug therapy (2nd ed.). Philadelphia, Pa., [etc.]: Lippincott Williams & Wilkins. p. 25. ISBN978-0-7817-8355-2 . Retrieved 2012-02-05 .
- ^ D.E. Golan, A.H Tashjian Jr, E.J. Armstrong, A.West. Armstrong. (2007) Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy Lippincott Williams & Wilkins ISBN 0-7817-8355-0
- ^ Surin A, Pshenichkin Due south, Grajkowska Due east, Surina E, Wroblewski JT (March 2007). "Cyclothiazide selectively inhibits mGluR1 receptors interacting with a common allosteric site for non-competitive antagonists". Neuropharmacology. 52 (3): 744–54. doi:x.1016/j.neuropharm.2006.09.018. PMC1876747. PMID 17095021.
- ^ Lipton SA (January 2004). "Failures and successes of NMDA receptor antagonists: molecular basis for the utilize of open up-channel blockers like memantine in the treatment of acute and chronic neurologic insults". NeuroRx. 1 (1): 101–10. doi:10.1602/neurorx.1.i.101. PMC534915. PMID 15717010.
- ^ Parsons CG, Stöffler A, Danysz W (November 2007). "Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic organization—as well fiddling activation is bad, too much is even worse". Neuropharmacology. 53 (vi): 699–723. doi:x.1016/j.neuropharm.2007.07.013. PMID 17904591. S2CID 6599658.
- ^ Principles and Practice of Pharmacology for Anaesthetists By Norton Elwy Williams, Thomas Norman Calvey Published 2001 Blackwell Publishing ISBN 0-632-05605-3
- ^ Patil PN (2002). "Everhardus J. Ariëns (1918–2002): a tribute". Trends Pharmacol. Sci. 23 (seven): 344–5. doi:10.1016/S0165-6147(02)02068-0.
- ^ Bosier B, Hermans Eastward (August 2007). "Versatility of GPCR recognition by drugs: from biological implications to therapeutic relevance". Trends in Pharmacological Sciences. 28 (8): 438–46. doi:x.1016/j.tips.2007.06.001. PMID 17629964.
- ^ Pulvirenti 50, Koob GF (April 2002). "Being partial to psychostimulant addiction therapy". Trends in Pharmacological Sciences. 23 (4): 151–3. doi:10.1016/S0165-6147(00)01991-10. PMID 11931978.
- ^ Vadivelu N, Hines RL (2007). "Buprenorphine: a unique opioid with broad clinical applications". Journal of Opioid Direction. 3 (1): 49–58. doi:10.5055/jom.2007.0038. PMID 17367094.
- ^ Greasley PJ, Clapham JC (December 2006). "Inverse agonism or neutral animosity at Thousand-protein coupled receptors: a medicinal chemistry challenge worth pursuing?". European Journal of Pharmacology. 553 (1–3): i–9. doi:10.1016/j.ejphar.2006.09.032. PMID 17081515.
- ^ Kenakin T (January 2004). "Efficacy as a vector: the relative prevalence and paucity of inverse agonism". Molecular Pharmacology. 65 (i): 2–11. doi:10.1124/mol.65.i.2. PMID 14722230. S2CID 115140.
- ^ Leurs R, Church MK, Taglialatela M (April 2002). "H1-antihistamines: changed agonism, anti-inflammatory deportment and cardiac furnishings". Clinical and Experimental Allergy. 32 (4): 489–98. doi:10.1046/j.0954-7894.2002.01314.10. PMID 11972592. S2CID 11849647.
- ^ Frang H, Cockcroft V, Karskela T, Scheinin One thousand, Marjamäki A (August 2001). "Phenoxybenzamine bounden reveals the helical orientation of the third transmembrane domain of adrenergic receptors". The Journal of Biological Chemistry. 276 (33): 31279–84. doi:10.1074/jbc.M104167200. PMID 11395517.
External links [edit]
-
 Media related to Receptor antagonists at Wikimedia Eatables
Media related to Receptor antagonists at Wikimedia Eatables
Which Is Incorrect About Inflammation,
Source: https://en.wikipedia.org/wiki/Receptor_antagonist
Posted by: sanderslawen1948.blogspot.com


0 Response to "Which Is Incorrect About Inflammation"
Post a Comment